Launch FirstinMedtech
Engineering intelligence
that gets your tech to market
Develop and iterate end-to-end
your medical device documentation.
Explore how reliable and fast it can be.
Streamline engineering and compliance work on
one integrated, intelligent platform.
Assistant
Your Expert, Always Available
AI copilot that guides you through design decisions and regulatory requirements with instant, context-aware support.
Workspace
Design and Document Together
Create compliant documentation with AI assistance while managing your complete product lifecycle with full traceability.
Vault
Quality Management Made Simple
Complete eQMS for managing CAPAs, complaints, NCRs, and audits with automated traceability across your quality system.
Workflows
Automate Your Process
Multi-model agents that automate compliance workflows from requirements through validation, delivering audit-ready work product.
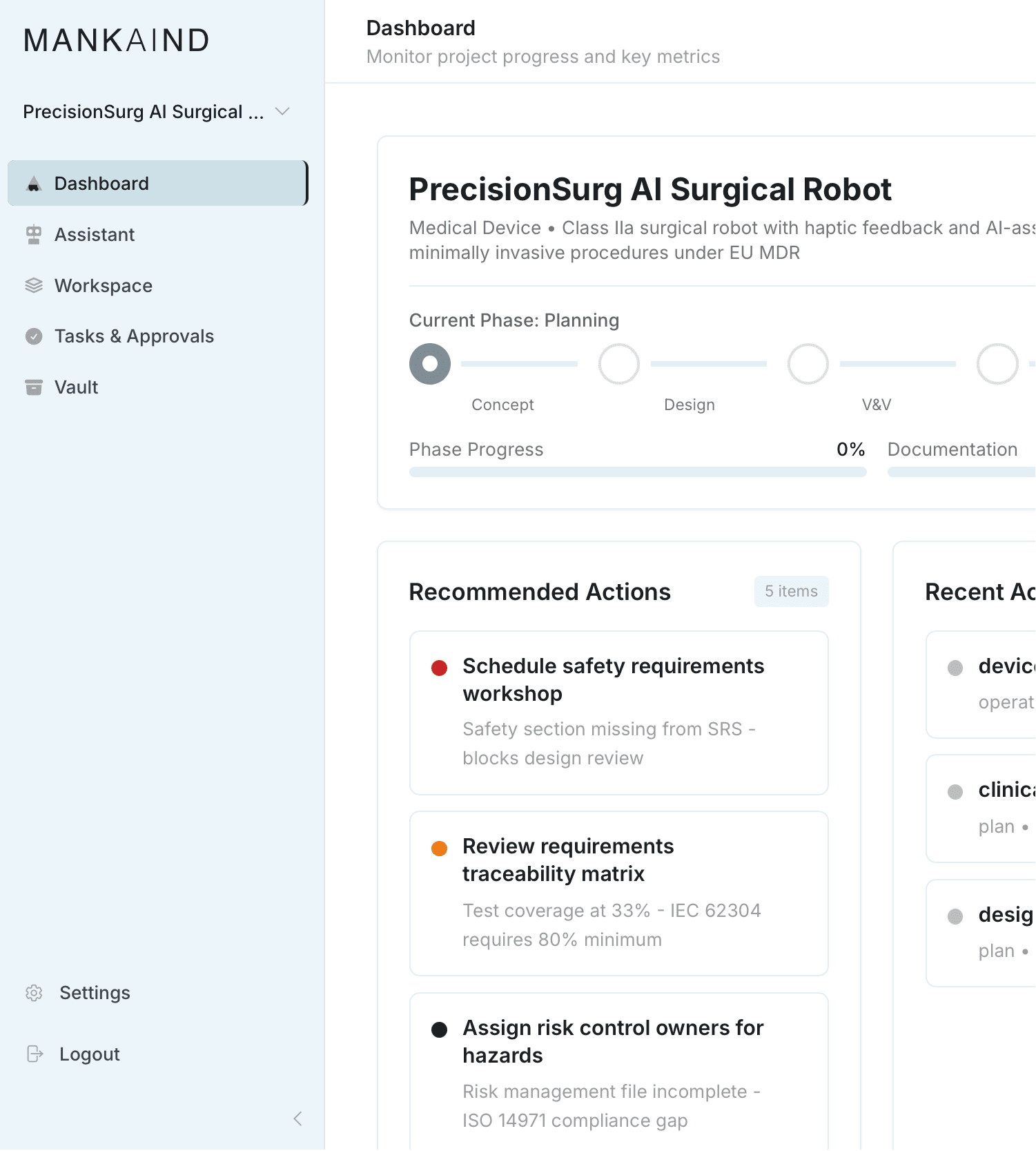
An Integrated
Development Environment
MANKAIND transforms how regulated industries
professionals build technology.
Security & Compliance
Enterprise-grade security with internationally recognized certifications.
SOC 2 Type II
Third-party security audits
ISO/IEC 27001:2022
Information security management
ISO/IEC 42001:2023
AI management system standard
AES-256 Encryption
Military-grade data protection
GDPR & CCPA
Global privacy compliance
Zero Retention
No data storage or training